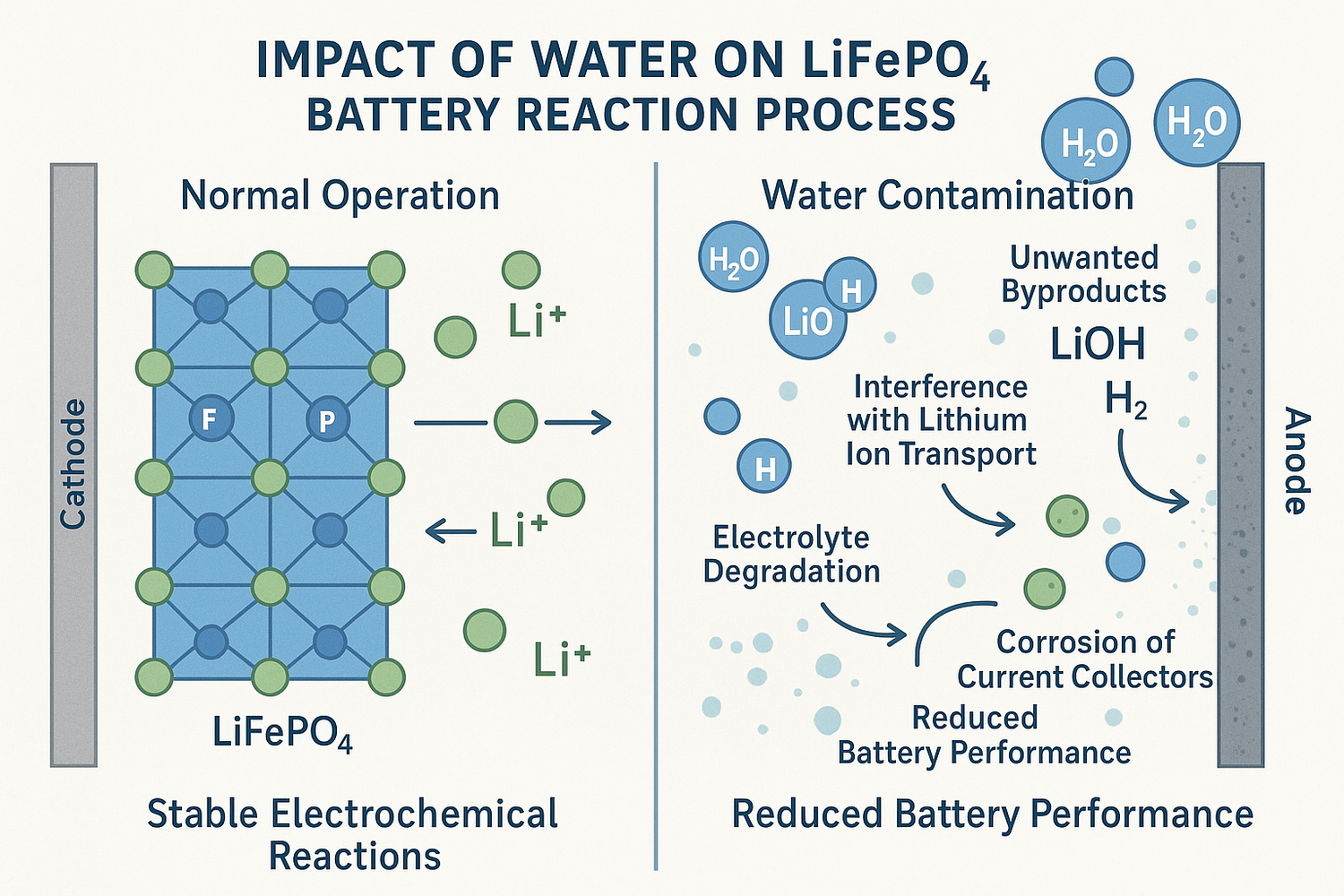
When LiFePO4 batteries experience water exposure, water seeps inside and interacts with internal components. The reaction process includes electrolyte dilution, acid formation, and hydrogen gas release. These reactions can damage electrodes, corrode terminals, and trigger swelling or rupture. Direct contact with water increases the risk of short circuit, overheating, or even explosion. Water exposure impacts battery performance and safety, especially in lifepo4 batteries. Although lifepo4 offers strong safety, careful handling remains essential.
Key Takeaways
- Water entering LiFePO4 batteries causes chemical reactions that damage internal parts and reduce battery performance.
- The electrolyte breaks down when exposed to water, producing corrosive acids and gases that harm the battery.
- Water exposure leads to capacity loss, shorter battery life, swelling, and physical damage.
- Short circuits, fire, explosion, and toxic gas release are serious safety risks from water-damaged batteries.
- Always disconnect and avoid charging batteries that have been exposed to water to prevent accidents.
- Proper disposal of water-damaged batteries as hazardous waste protects people and the environment.
- Using waterproof cases, sealed housings, and storing batteries in dry places helps prevent water damage.
- Regular inspection and careful handling in wet or humid environments keep LiFePO4 batteries safe and reliable.
Lithium Iron Phosphate Battery Structure

Cell Components
Lithium iron phosphate batteries, often called lifepo4 batteries, feature a unique internal structure that sets them apart from other lithium batteries. Each battery consists of four main components:
- Cathode: Made from lithium iron phosphate, this part serves as the source of lithium ions during discharge.
- Anode: Typically composed of graphite, the anode stores and releases lithium ions as the battery cycles.
- Electrolyte: This liquid contains lithium salts dissolved in an organic solvent, enabling ion transfer between the cathode and anode.
- Separator: A thin barrier that prevents direct contact between the cathode and anode, reducing the risk of short circuits while allowing lithium ions to pass.
Scientific studies using in situ neutron diffraction reveal that both the cathode and anode undergo structural changes as lithium ions move during charging and discharging. These changes are essential for the battery’s function and performance. However, when water intrusion occurs, these internal components become vulnerable to chemical reactions that can compromise the battery’s stability.
Electrolyte Role
The electrolyte in lifepo4 batteries plays a critical role in enabling the movement of lithium ions between the electrodes. At the molecular level, the electrolyte is sensitive to water exposure. Water molecules can permeate the carbon coating on lithium iron phosphate particles, which is not a perfect barrier. This allows water to reach the active material, causing rapid chemical reactions on the particle surface. Lithium ions react with water, leading to the formation of lithium hydroxide and lithium carbonate. These reactions can extract lithium from the structure, resulting in a loss of capacity and performance.
Surface modifications, such as adding hydrophobic groups, can increase the water contact angle and reduce the interaction between water and lithium ions. This approach helps limit water-induced degradation, improving the stability and lifespan of lifepo4 batteries. Despite these advances, the electrolyte remains one of the most vulnerable parts of the battery when exposed to water.
Water Resistance Features
Lifepo4 batteries incorporate several design features to enhance water resistance. Many batteries achieve an IP68 waterproof rating, which indicates a high level of protection against water intrusion. Manufacturers use single cell containers made from fire-retardant and stable materials to improve durability. These containers help prevent water from reaching sensitive internal components. Additional protective measures include sealants, coatings, and encapsulation, all designed to block water and maintain battery safety.
Note: While the lithium iron phosphate cathode itself shows some resistance to water, other components—especially the electrolyte and separator—remain susceptible to damage from water exposure. Proper design and manufacturing practices are essential to ensure the long-term reliability of lifepo4 batteries in environments where water intrusion is a risk.
Reaction Process After Water Exposure
Electrolyte Decomposition
The reaction process in lifepo4 batteries begins immediately after water exposure. Water molecules penetrate the battery casing and reach the electrolyte, which typically contains lithium hexafluorophosphate (LiPF6). The reaction to water initiates a series of water reactions that break down the electrolyte. LiPF6 reacts with water to form hydrofluoric acid (HF) and phosphorus oxyfluoride (POF3), both of which are highly corrosive. This hazardous reaction not only damages the electrolyte but also attacks the cathode and anode materials.
The rate of electrolyte decomposition depends on temperature and the presence of water. The following table summarizes how water contamination accelerates iron (Fe) dissolution from the cathode, which directly impacts battery stability and performance:
|
Condition |
Temperature |
Fe Loading on Graphite Electrode (μg/cm²) |
Notes |
|---|---|---|---|
|
Control electrolyte, dried at 100 °C |
40 °C |
5.5 |
Significant Fe dissolution due to water contamination |
|
Control electrolyte, dried at 120 °C |
40 °C |
0.2 |
Removing water drastically reduces Fe dissolution |
|
Control electrolyte, dried at 100 °C |
55 °C |
2.2 |
Fe dissolution present but less than at 40 °C |
|
Control electrolyte, dried at 120 °C |
55 °C |
1.8 |
Slight reduction in Fe dissolution with water removal |
|
Electrolyte additives (various) |
20 °C |
~0 |
Virtually no Fe dissolution after ~2500 h cycling |
|
Electrolyte additives (various) |
40 °C |
Much lower than control |
Additives suppress Fe dissolution regardless of water content |
|
Electrolyte additives (various) |
55 °C |
Much lower than control |
Small reduction with water removal, but overall low Fe dissolution |
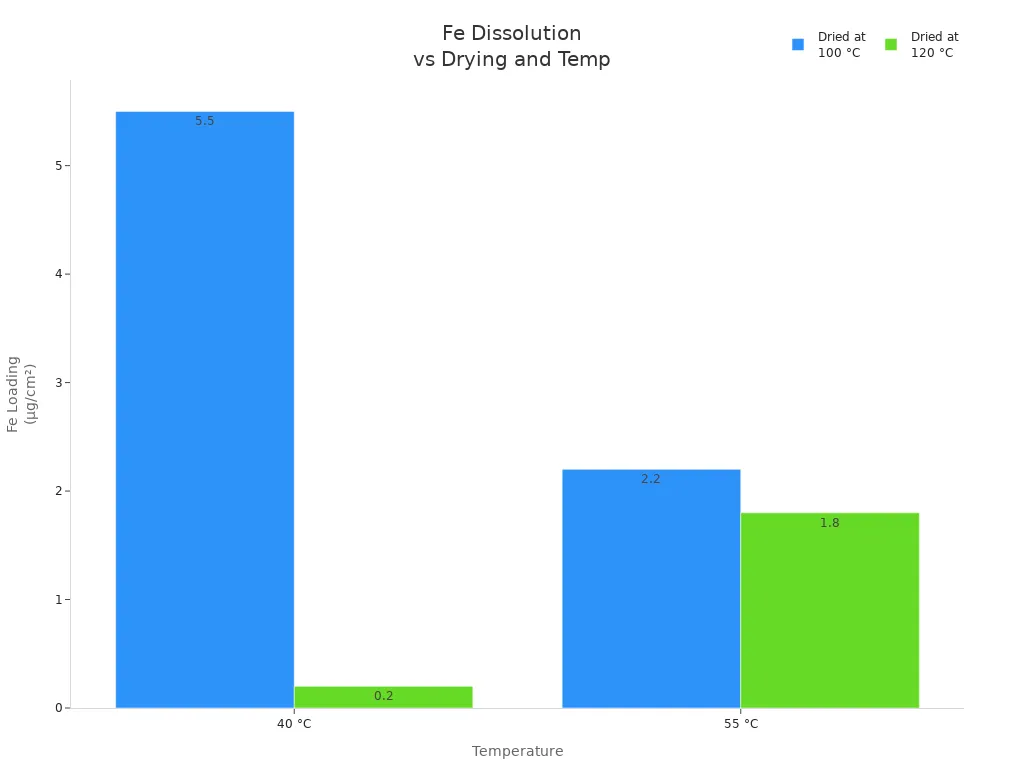
This data shows that even trace amounts of water can trigger significant decomposition in lithium battery electrolytes. Removing water or using additives can greatly reduce these effects, but lifepo4 batteries remain vulnerable if exposed.
Gas and Heat Generation
The reaction process after water exposure also produces gases and heat inside lithium batteries. When water reacts with the electrolyte, hydrogen gas (H2) forms as a byproduct. The buildup of hydrogen gas increases internal pressure, which can cause the battery to swell or rupture. In addition, the formation of hydrofluoric acid and other corrosive compounds releases heat. This heat can accelerate further water reactions, leading to a dangerous cycle of gas and heat generation.
Lifepo4 batteries, like other lithium batteries, face increased risks of swelling, venting, or even explosion if gas production becomes excessive. The combination of heat and gas not only threatens the physical integrity of the battery but also increases the risk of fire, especially if the battery casing becomes compromised.
Corrosion and Coating Formation
Corrosive compounds formed during the reaction process attack multiple components within lifepo4 batteries. Hydrofluoric acid and phosphorus oxyfluoride degrade the cathode coating, obstructing electron flow and reducing power output. The anode, usually made of graphite, suffers from pore clogging, which lowers its effectiveness. The separator, designed to keep the electrodes apart, absorbs water and swells, sometimes cracking or puncturing. This loss of barrier function increases the risk of short circuits.
Metal casings corrode and weaken, especially in saltwater environments, where higher conductivity accelerates the reaction to water. Over time, these water reactions lead to capacity loss, swelling, and even short circuits. Toxic fumes and leaked chemicals can escape, posing health and environmental hazards. The overall stability of the battery declines as corrosion spreads, and the risk of fire or explosion rises.
Note: Water exposure in lifepo4 batteries triggers a chain of hazardous reactions that compromise internal stability and safety. The reaction process not only reduces battery performance but also introduces significant safety risks, especially in environments with high humidity or saltwater.
Effects on Battery Performance
Capacity Loss
Water exposure leads to a rapid decline in the capacity of lithium batteries. When water enters a lifepo4 battery, chemical reactions begin to degrade the electrolyte and active materials. These reactions reduce the number of lithium ions available for charge and discharge cycles. As a result, the battery cannot store as much energy as before. Users may notice that devices powered by these batteries run for shorter periods between charges.
The following table summarizes the main factors contributing to capacity loss in lifepo4 batteries after water exposure:
|
Factor |
Description |
Impact on Capacity |
|---|---|---|
|
Electrolyte decomposition |
Water breaks down the electrolyte |
Reduces ion mobility |
|
Cathode corrosion |
Water reacts with lithium iron phosphate |
Lowers active material |
|
Anode pore clogging |
Byproducts block lithium movement |
Limits charge storage |
Note: Even a small amount of water can cause irreversible damage to battery performance. The loss of capacity often becomes permanent, making recovery difficult.
Cycle Life Reduction
Lifepo4 batteries are known for their long cycle life. However, water exposure shortens this lifespan significantly. Each charge and discharge cycle after water damage accelerates the breakdown of internal components. Corrosion, gas formation, and separator swelling all contribute to faster aging.
The potential consequences of repeated cycling in a water-damaged lithium battery include:
- Increased internal resistance, which leads to heat generation.
- Accelerated loss of active lithium, reducing usable cycles.
- Greater risk of short circuits, which can end the battery’s service life abruptly.
Manufacturers design lithium batteries to withstand hundreds or even thousands of cycles. Water damage undermines this durability, resulting in fewer usable cycles and earlier replacement.
Swelling and Physical Damage
Physical changes often signal serious consequences after water exposure. Lifepo4 batteries may swell due to gas buildup inside the cell. This swelling can deform the battery casing and put pressure on internal layers. In severe cases, the casing may crack or rupture, exposing hazardous materials.
Common signs of swelling and physical damage include:
- Bulging or misshapen battery packs.
- Leakage of electrolyte or other chemicals.
- Unusual odors or discoloration on the battery surface.
These physical effects not only indicate damage to battery performance but also increase safety risks. Swollen lithium batteries may no longer fit securely in devices, and damaged casings can lead to short circuits or fire hazards.
⚠️ Caution: Never attempt to use or recharge a swollen lithium battery. The potential consequences include fire, toxic gas release, and further damage to connected devices.
Water exposure creates a chain reaction of chemical and physical changes in lifepo4 batteries. The damage to battery performance, cycle life, and physical integrity highlights the importance of keeping lithium batteries dry and protected from water damage.
Safety Risks of Water Exposure
Short Circuit
Water exposure introduces significant safety risks to lithium iron phosphate batteries. When water enters the battery, it can bridge the gap between the anode and cathode. This process creates a direct path for electrical current, resulting in short circuits. Saltwater increases this risk because it acts as a strong conductor. The presence of salt accelerates chemical reactions, which can lead to the rapid formation of acidic compounds and flammable gases.
Short circuits inside batteries generate heat. This heat can cause swelling, leakage, and even thermal runaway. If the battery casing becomes compromised, the risk of fire hazards increases. Manufacturers design batteries with sealed, waterproof casings and assign IP ratings, such as IP67, to reduce the chance of water ingress. However, prolonged exposure or submersion can still allow water to reach internal components. Users should disconnect wet batteries immediately and avoid charging them to prevent further damage.
Tip: Never attempt to recharge a battery that has been submerged or shows signs of swelling. Charging can worsen short circuits and increase the risk of overheating.
Fire and Explosion
Short circuits and chemical reactions inside water-exposed batteries can lead to fire and explosion. When water reacts with the organic electrolyte, it produces heat and flammable gases. The buildup of hydrogen gas inside the battery increases internal pressure. If the pressure exceeds the strength of the casing, the battery may rupture or explode.
Fire hazards become more severe if the battery is damaged or improperly handled. Corrosion of internal metallic components weakens the structure, making it easier for heat and gas to escape. In some cases, even a small spark can ignite the flammable gases released during these reactions. Proper safety measures, such as using batteries with high waterproof ratings and inspecting for damage, help reduce these risks.
- Common fire hazards from water-exposed batteries:
- Overheating due to short circuits
- Ignition of flammable gases
- Rupture of the battery casing
Toxic Gas Release
The release of toxic chemicals represents another major safety concern. When lithium iron phosphate batteries undergo thermal runaway or fire, they emit hazardous gases. Hydrogen fluoride (HF) and phosphorus oxyfluoride (POF3) are the primary toxic gases released. Fire tests show that HF can be produced in quantities ranging from 20 to 200 mg/Wh, while POF3 appears at lower levels. These gases irritate the respiratory tract and can cause chemical burns if they come into contact with skin or eyes.
|
Toxic Gas |
Conditions |
Quantity Released (mg/Wh) |
|---|---|---|
|
Hydrogen Fluoride (HF) |
Fire with/without water mist |
20 - 200 |
|
Phosphorus Oxyfluoride (POF3) |
Detected at 0% SOC in some batteries |
15 - 22 |
Water exposure alone may not release large amounts of toxic gases unless combined with fire or overheating. However, any release of toxic chemicals poses health and environmental risks. First aid for exposure includes flushing eyes with water, washing skin thoroughly, and seeking medical attention if symptoms persist.
⚠️ Caution: Always handle batteries with care after water exposure. Toxic gases and chemical burns can occur if the battery leaks or ruptures.
Handling Wet Batteries
Immediate Actions
When lithium iron phosphate batteries come into contact with water, immediate action is essential to protect both people and property. The first priority centers on safety. Individuals should always wear protective gloves before touching any leaking or damaged battery. This precaution helps prevent skin irritation or burns from chemicals that may have leaked. Keeping ignition sources away from the area is crucial, as chemical reactions inside the battery can release flammable gases.
A well-equipped emergency kit should be available. This kit includes gloves, face masks to avoid inhaling toxic fumes, absorbent materials for cleaning, and baking soda to neutralize any electrolyte leaks. A fire extinguisher should remain close at hand in case a fire starts. If a battery appears swollen or heavily damaged, it should be disposed of immediately to prevent rupture or explosion. Regular inspection and proper storage of batteries help reduce the risk of water exposure and related hazards.
⚠️ Safety Tip: Always prioritize personal safety and environmental protection when handling wet batteries.
Inspection Steps
After ensuring immediate safety, a careful inspection of the battery is necessary. The following steps outline what to do:
- Avoid direct contact with the battery. Use insulated gloves and eye protection.
- Disconnect the battery from any devices or chargers if it is safe to do so.
- Do not attempt to use or recharge the battery after water exposure.
- Move the battery to a safe, non-flammable area, preferably outdoors. Observe from a distance for signs of swelling, heat, or smoke.
- If the battery remains stable, place it in a fire-resistant container, such as a metal bucket filled with sand. Ensure the container is ventilated and not sealed airtight.
- Inspect the battery’s seals, enclosures, and terminals for any visible damage or wear.
- Contact the manufacturer or a hazardous waste facility for professional advice on disposal.
- Dispose of the battery according to local hazardous waste regulations. Never throw it in regular trash.
These steps help prevent further damage and reduce the risk of fire or toxic gas release.
Drying and Recovery
Drying and recovery of lithium iron phosphate batteries after water exposure require controlled conditions. The most effective method involves a selective hydrometallurgical process. This process uses sodium persulfate as an oxidizing agent to leach lithium while precipitating iron as FePO4. After leaching, the filter residue is washed several times with deionized water. The residue then dries in an oven at 80°C for 12 hours. This drying step is critical for preparing the material for further processing.
Calcination at 650°C for 5 hours follows, which removes carbon from the residue. Lithium ions in the filtrate are recovered by adding sodium carbonate powder at 90°C, causing lithium carbonate to precipitate. This compound is then filtered and washed with hot water. The process achieves a high lithium recovery rate and avoids harsh acid conditions, making it environmentally friendly. Controlled oven drying at 80°C for 12 hours stands out as an effective way to prepare battery materials for recovery after water exposure.
Note: Only professionals should attempt recovery processes. Most users should focus on safety and proper disposal rather than attempting to dry or restore wet batteries themselves.
Disposal Guidelines
Proper disposal of lithium iron phosphate batteries exposed to water is essential for safety and environmental protection. These batteries, once compromised, become hazardous waste. They require careful handling and must never enter regular trash or curbside recycling.
-
Classify as Hazardous Waste
Lithium iron phosphate batteries fall under hazardous waste regulations. Local authorities and environmental agencies treat them as such due to the risk of toxic gas release and chemical contamination. -
Isolate Terminals
Before disposal, cover each battery’s terminals with non-conductive tape. This step prevents accidental short circuits. Alternatively, place each battery in a separate plastic bag. Isolation reduces the risk of fire during storage and transport. -
Separate Damaged Batteries
Store water-exposed or damaged batteries away from undamaged ones. Use containers made of non-conductive materials. Label these containers clearly to indicate hazardous contents. -
Choose Proper Storage Locations
Place compromised batteries in cool, dry, and well-ventilated areas. Keep them away from flammable materials and direct heat sources. Avoid storing batteries in occupied spaces to minimize exposure to toxic gases if a leak occurs. -
Use Certified Collection Sites
Take batteries to certified battery collection points or household hazardous waste facilities. These sites follow strict protocols for handling, storage, and disposal. They help prevent environmental contamination and reduce health risks. -
Avoid Transport by Air
Damaged lithium batteries may not travel by air due to strict transportation regulations. If transport is necessary, follow Department of Transportation packaging requirements. -
Do Not Attempt Repairs
Never open, repair, or dismantle compromised batteries. Internal chemicals can cause burns or release toxic gases. -
Note: Water-damaged lithium iron phosphate batteries can emit hazardous gases such as hydrogen fluoride if they catch fire or overheat. Water used to suppress battery fires may create toxic runoff, contaminating soil and groundwater. Proper disposal prevents these environmental hazards.
-
Employee and Facility Safety
Facilities handling large quantities of damaged batteries should train staff, install advanced fire detection and suppression systems, and coordinate with emergency responders. Frequent inspections help maintain a safe environment.
By following these disposal guidelines, individuals and organizations protect themselves, their communities, and the environment from the dangers associated with water-compromised lithium iron phosphate batteries.
Prevention and Protection
Waterproofing Methods
Manufacturers and users employ several methods to waterproof a battery pack and enhance its resistance to water exposure. The choice of technology depends on the application, cost, and required protection level. The following table summarizes common waterproof sealing technologies:
|
Waterproof Sealing Technology |
Advantages |
Disadvantages |
Applicable IP Rating |
|---|---|---|---|
|
O-Ring Sealing |
Low cost, easy replacement |
Wears out over time |
IP67/IP68 |
|
Laser Welding |
Fully sealed, highly reliable |
High cost, non-repairable |
IP68/IP69K |
|
Encapsulation Resin |
Shockproof, corrosion-resistant |
Poor heat dissipation, not removable |
IP68 |
To waterproof a battery pack, users can select housings with built-in waterproof features or apply waterproof coatings. Waterproof adhesive strips or rubber seals help prevent moisture ingress. Protective cases or sleeves shield batteries from both moisture and physical impacts. Potting or encapsulating batteries with insulating resin creates a sealed barrier, while electrode insulation, especially at the cathode, improves reliability. Regular inspection of seals and housings ensures that protective measures remain effective.
Tip: Choosing the right IP rating, such as IP67 for short-term immersion or IP68 for long-term submersion, helps match the level of waterproofing to the intended environment.
Storage Tips
Proper storage plays a crucial role in preventing water intrusion and extending the lifespan of lithium iron phosphate batteries. Users should store batteries in dry, secure locations away from any potential water exposure. Battery compartments must remain properly sealed to block moisture. Waterproof cases provide added protection when storing or transporting batteries in moist environments.
- Store batteries in dry places, avoiding humidity and flooding.
- Use waterproof battery cases for outdoor or marine storage.
- Inspect seals and enclosures periodically for signs of wear or damage.
- Protect batteries from rain, wind, and extreme temperatures.
- Consider coatings such as urethane, silicone, or rubberized paints to waterproof a battery pack, ensuring terminals stay accessible.
Marine-grade waterproof boxes allow charging and power draw without removing the battery, offering convenience and safety. Users should avoid submerging batteries or exposing them to excessive moisture. Regular checks for leakage or corrosion help detect early signs of water damage.
Maintenance in Humid Environments
Maintaining lithium iron phosphate batteries in humid environments requires consistent attention to protective measures. Users should avoid high humidity and moisture exposure to protect both cells and enclosures. Proper ventilation in battery enclosures prevents moisture buildup. Waterproof cases or devices with good sealing minimize water ingress during outdoor use.
- Regularly inspect batteries for corrosion or external damage.
- Store unused batteries in cool, dry locations with controlled temperatures between 10°C and 30°C.
- Monitor temperature and connections frequently to maintain battery health.
- Do not charge batteries if they are wet, as this can cause short circuits.
- If a battery contacts water, disconnect power, remove the battery, dry it in a ventilated area, and seek professional inspection before reuse.
Storing batteries with a partial charge (50–70%) in cool, dry environments helps preserve their performance. Built-in Battery Management Systems (BMS) monitor temperature, voltage, and current, further supporting safe operation. Periodic inspections for voltage and wear ensure that batteries remain reliable, even in challenging conditions.
Common Water Exposure Scenarios
Marine Use
Marine environments present unique challenges for lithium iron phosphate batteries. Boating activities, such as sailing or kayaking, often involve direct contact with water. Batteries may experience splashing or even full submersion during capsizing or water ingress. Saltwater exposure significantly increases the risk of damage. Salt ions accelerate chemical reactions inside the battery, leading to rapid corrosion, swelling, and capacity loss. Hydrogen gas and acidic compounds form more aggressively in saltwater, raising the risk of short circuits and explosions. Water can enter through vulnerable points like battery terminals, box joints, or vents. Corrosion at the terminals weakens electrical connections and increases the chance of overheating. Marine batteries typically offer water resistance, but not complete waterproofing. Proper waterproof battery boxes, protective casings, and regular maintenance help reduce these risks.
⚠️ Note: Saltwater exposure can cause toxic leaks and environmental hazards. Specialized waterproofing and careful handling are essential in marine settings.
Outdoor and Emergency
Outdoor and emergency situations expose lithium iron phosphate batteries to unpredictable conditions. Rain, flooding, and accidental submersion can all lead to water ingress. Even though many batteries appear sealed, cracks or wear over time allow water to penetrate. Water dilutes the electrolyte, causing chemical reactions that produce corrosive acids. These acids damage electrodes and separators, leading to reduced performance and increased safety risks. Saltwater, in particular, accelerates corrosion and can cause internal short circuits. Outdoor use also exposes batteries to dust and moisture, which can degrade the casing and internal structure. Emergency scenarios, such as rescue operations or severe weather, may involve rapid submersion or seawater exposure. Protective measures, including waterproof cases and IP-rated enclosures, help prevent damage during these water exposure scenarios.
- Water can enter through cracks or worn seals.
- Saltwater causes electrode peeling and separator damage.
- Charging after water exposure increases explosion risk.
- Waterproof covers and high IP ratings provide added protection.
Flooding Events
Flooding events pose a significant threat to lithium iron phosphate batteries. Rising water levels can submerge batteries for extended periods. Both freshwater and saltwater flooding can cause severe internal damage. Water bridges the gap between terminals, increasing the risk of short circuits and overheating. Floodwaters often contain contaminants that further accelerate corrosion and chemical breakdown. Battery casings may rust or deform, leading to electrolyte leakage and reduced safety. In residential or commercial settings, flooding can damage backup power systems, solar storage units, and electric vehicles equipped with these batteries. Immediate removal from water, thorough inspection, and professional assessment are critical after any flooding event.
💡 Tip: Store batteries above ground level and use waterproof enclosures in flood-prone areas to minimize risk.
Water exposure can cause irreversible damage to lithium iron phosphate batteries, including capacity loss, swelling, and increased fire risk. Quick action and proper handling remain essential for safety. Professionals recommend storing batteries in dry places, using protective cases, and never charging wet batteries. The table below highlights the effectiveness of water mist in fire suppression for these batteries:
|
Aspect |
Water Mist Effectiveness |
|---|---|
|
Fire Suppression |
Most effective, prevents reignition |
|
Cooling Capability |
Continuous cooling, reduces fire intensity |
LiFePO4 batteries offer strong safety, but users should always exercise caution and consult experts if damage occurs.
FAQ
What happens if a LiFePO4 battery gets wet?
Water can enter the battery and trigger chemical reactions. These reactions may cause corrosion, gas formation, and capacity loss. The battery may swell or leak, and safety risks increase.
What signs indicate water damage in a LiFePO4 battery?
Common signs include swelling, leakage, unusual odors, and visible corrosion. The battery may also lose capacity or fail to hold a charge. Users should inspect for these symptoms after suspected water exposure.
What should users do if a LiFePO4 battery is exposed to water?
Users should disconnect the battery from devices, avoid charging, and move it to a safe area. Protective gloves are recommended. Contact a professional for inspection or disposal.
What risks does water exposure create for battery safety?
Water exposure increases the risk of short circuits, fire, and toxic gas release. The battery may overheat or rupture. Proper handling and quick action reduce these hazards.
What makes LiFePO4 batteries more water-resistant than other lithium batteries?
The lithium iron phosphate cathode resists water better than other chemistries. Manufacturers use sealed casings and protective coatings. However, the electrolyte and other parts remain vulnerable.
What environments pose the highest risk for water exposure?
Marine, outdoor, and flood-prone environments present the greatest risks. Saltwater accelerates corrosion and chemical reactions. Users should use waterproof cases and inspect batteries regularly in these settings.
What disposal steps should users follow for water-damaged LiFePO4 batteries?
Users should treat water-damaged batteries as hazardous waste. Cover terminals, store in non-conductive containers, and take them to certified collection sites. Never throw them in regular trash.
What preventive measures help protect LiFePO4 batteries from water damage?
Waterproof cases, proper storage, and regular inspections help prevent water damage. Users should avoid charging wet batteries and keep them in dry, secure locations.